Angelo Giambrone, 12/03/2021
Healthcare facilities are committed to delivering high-quality care in a safe environment where patients should be protected from avoidable harm[1]. One such area in healthcare that is paramount in protecting patients is the decontamination function provided by the Central Sterile Services Department (CSSD). Effective sterilisation is a crucial element in the running of a hospital, ensuring operations can be safely performed and that patient wellbeing is maintained. Angelo Giambrone, Healthcare business development manager at Spirax Sarco UK, explores how decontamination leads and authorising engineers in Healthcare facilities can drive improvements in steam quality to deliver reliable and consistent sterilisation of reusable medical devices and touches on what the future may hold for the sterile services department in a decarbonising world.
Mitigating Healthcare-associated infections (HCAI)
Vulnerable hospital patients, often with compromised immune systems, are unfortunately at increased risk of infection. One report in the British Medical Journal stated that between 2016 and 2017[2], there were an estimated 653 000 healthcare-associated infections (HCAIs) among the 13.8 million adult inpatients in NHS general and teaching hospitals in England, of which 22 800 patients died as a result of their infection.
As such, HCAIs are closely monitored and this of course has been particularly scrutinised in 2020 with the ongoing efforts to minimise the spread of COVID-19. The National Institute for Health and Care Excellence (NICE), reported in 2016[3] that “the six most common types of healthcare-associated infections, which accounted for more than 80% of all healthcare-associated infections, were pneumonia and other respiratory infections (22.8%), urinary tract infections (17.2%), surgical site infections (15.7%), clinical sepsis (10.5%), gastrointestinal infections (8.8%) and bloodstream infections (7.3%)”.
With respect to surgical site infections, there is guidance on the preparation of the surgical site, procedures during the operation and post-operation care of the wound. One critical area in minimising risk of surgical site infection is in the effective decontamination of surgical equipment, ensuring that the equipment used in surgery is sterilised to the standards required by the industry, to help deliver the best possible outcome for the patients.
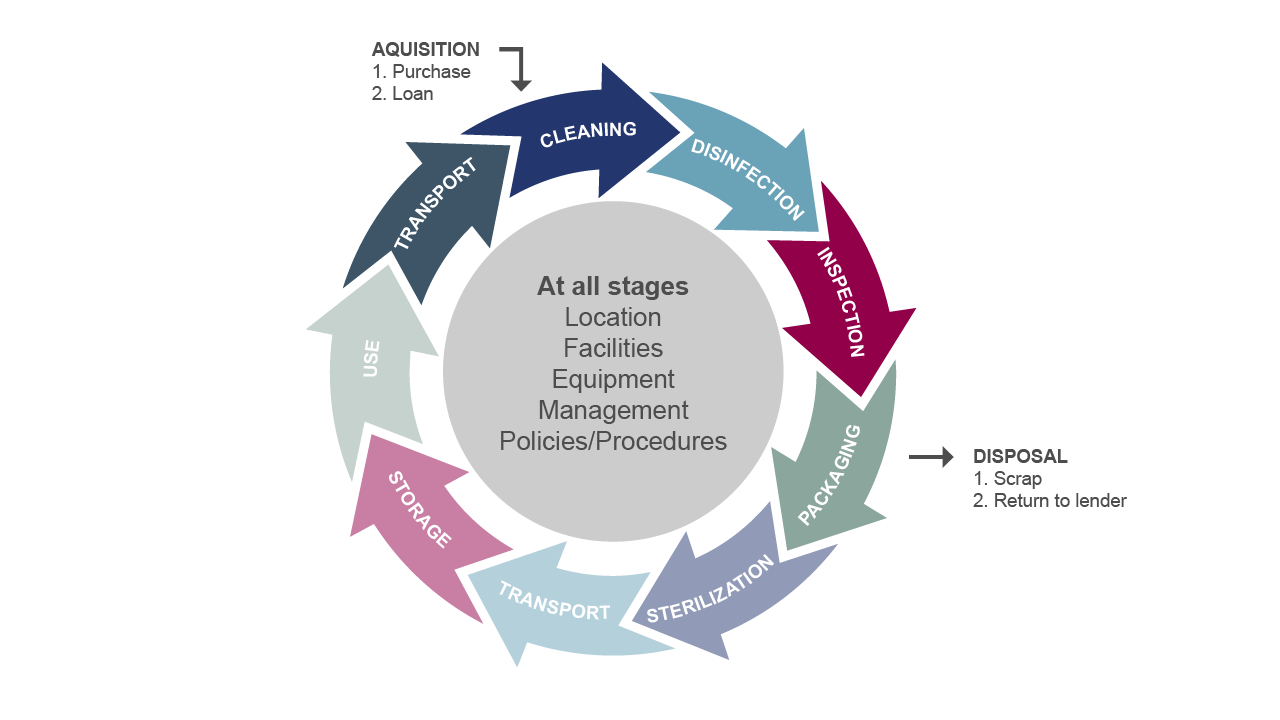
As a key infection control measure, decontamination is an umbrella term used to describe the process of cleansing to remove contaminants such as micro-organisms, making equipment safe for reuse in operating theatres, clinics, A&E departments and wards. There are three levels of decontamination[4] within the healthcare environment; the first line of defence is general cleaning, the second is disinfection and finally, the third is sterilisation for the removal of viable infectious agents including viruses and bacterial spores. Effective decontamination can be achieved by applying the appropriate actions at all stages of the decontamination life cycle process [Fig 1].
Figure 1 The decontamination life cycle
In this feature we will focus on the sterilisation element of the decontamination cycle (Fig.1). Steam provides the most reliable and efficient method of achieving effective sterilisation, which is why the healthcare industry standard for sterilisation is to use steam, delivered at a specific pressure and temperature. It is a simple, fast and safe way to decontaminate reusable medical devices such as surgical instruments. However it is important to consider how the steam is produced and delivered to the sterilisers in order to ensure that the quality standards are met and to achieve a successful and repeatable outcome from every sterilisation cycle.
The time taken for a cycle can depend on the steriliser and its contents. Another factor is the steam pressure. Steam temperature varies with pressure and Health Technical Memorandum (HTM) 01-01 Part C indicates how steriliser holding times vary with steam temperature, in order to achieve sterilisation. Optimising sterilisation cycle times of course has an impact on departmental efficiency, economy and most importantly, patient safety. Whatever steam pressure or cycle time is used, underpinning it all is the provision of high quality steam into the sterilisation process, to ensure the desired quality of the end product is reached.
Steam for safe sterilisation
A recent global study of sterilisation facilities conducted by Spirax Sarco aimed to understand the key processing barriers to delivering successful sterilisation outcomes. One issue seen repeatedly around the world was the presence of ‘wet packs’, together with the associated actions of dealing with and establishing the root cause of the problem. The phenomenon is recognised and explained in HTM 01-01 4.113 “wet spots or patches on the packaging show that liquid water has been drawn into the chamber. There are several possible explanations, including: a. poorly draining steam traps between the sterilizer and boiler (a sudden demand for steam can draw water out of a full trap); b. severe pressure fluctuations in the main; c. priming of the boiler leading to carry-over of water in the steam”. In addition to causes such as overloading of the steriliser, it is evident that poor quality steam, outside the specification detailed in BS EN 285, is a likely factor.
If there is evidence of wetness on the packaging when removed from the sterilisers, typically highlighted by the presence of darkened patches, the pack will need to have the damp wrapping removed and replaced with new. Then the pack will have to be put through the sterilisation process again. This of course could affect more than one pack in a batch. In addition to the reprocessing, there will be time and effort spent on establishing the cause of the problem. Key stakeholders within CSSDs can mitigate against increased processing times and costs, not to mention increased patient risk, by ensuring that the sterilisation process is not impeded by poor quality steam. The use of high-quality steam is emphasised in HTM 01-01. By using specific steam-to-steam generators that use a high quality water supply (typically Reverse Osmosis), an HTM-compliant header and a well-designed distribution system, steam quality can be best improved to reach a dryness level above 95% as stipulated in BS EN 285. Not only can the correct clean steam generator installation have an impact on dryness levels, but it also deals with reducing the risk of introducing contaminants into the sterilisation process.
But surely steam is clean, wherever it comes from? Well, the evaporation process certainly gives steam a broad element of ‘cleanliness’, but when we consider direct use applications such as pharmaceutical manufacturing, food processing or hospital sterilisation, there are in fact different grades of steam, which are influenced by the water quality used and the materials and design of the generation plant and distribution system. This all influences the purity of the steam, which can be measured by the level of contaminants present in the condensate that is formed.
Clean Steam Generators (often referred to as Chemical Free Steam Generators) use regular plant steam as the primary thermal energy source to boil a clean water supply (such as reverse osmosis water) into steam that is free of harmful substances and impurities. The heat exchange is indirect via a steam heating coil, so the plant steam never comes into direct contact with the clean steam. This high-quality steam can then be applied directly to the sterilisation process. Clean steam generators can help CSSD departments take a quality and patient first approach in the sterilisation of reusable medical devices. Fundamentally, sterilisation must be right first time, every time to safeguard patient health and ensure sterile services run as smoothly as possible. By implementing a CSG system that delivers dedicated high-quality steam for the sterilisation process, healthcare facilities can better control the quality of their steam supply to protect against ineffective sterilisation.
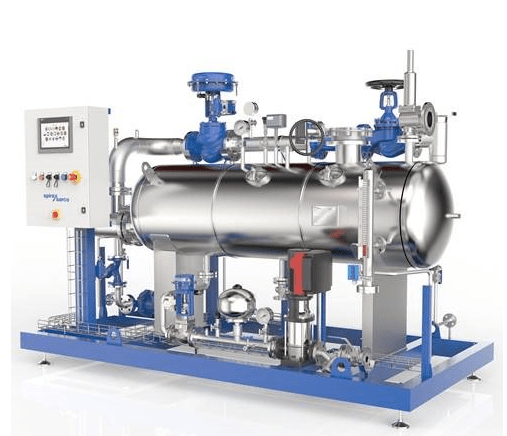
The Spirax Sarco clean steam generator (Figure 2) for Healthcare has been specifically developed for sterilisation applications, with the aim to deliver steam of a quality that exceeds the standards set out in BS EN 285. This latest unit draws on the experience of decades in the manufacture of clean steam generators and implements innovative thinking in relation to degassing of the water. The result is a unit that delivers repeatable high-quality steam under a variety of operating conditions which exceeds the minimum steam dryness value of 95%, as well as reducing level of non-condensable gases (as outlined in BS EN 285).
Steriliser operation has a particularly ‘peaky’ steam load profile, with the load high at certain points of the cycle and then instantaneously dropping to low flow rates at other points of the cycle. The latest monitoring equipment utilised by Spirax Sarco shows that the dryness output from their latest generator exceeds the BS EN 285 recommendations throughout these dynamic changes in load, ensuring that high quality is delivered continuously throughout the sterilisation cycle.
When trying to trace the cause of wet packs on existing plant, this is something that is particularly difficult to assess. The common dryness testing regime is based on a point in time and is not continuous, so identifying wet steam can be somewhat ‘hit and miss’ and dependant on operating conditions prevailing at the time of the test. This latest generator can therefore safeguard against issues resulting from poor steam quality at all points in the operating cycle.
Figure 2 Dedicated clean steam generator systems for healthcare settings can be used to better control the quality of the steam supply to ensure reliable and consistent sterilisation of reusable medical devices
The Future of Clean Energy
We are all aware of the drive to reduce the use of fossil fuels in our bid to lower carbon emissions. The way this impacts on the thermal needs of industry is a problem that is challenging our engineering minds, particularly where there are large instantaneous thermal loads required by the process.
The CSSD is one area that faces this challenge. Typically a hospital or CSSD department will have an energy centre that uses gas as the primary fuel, which then distributes thermal energy in the form of steam to the sterile services plant. The steam has very high energy content when compared to a typical circulating hot water system (26 times more energy per kg than a water circuit working on 80-60oC flow and return). Steam is also very easily distributed, without the need for circulation pumps to move large volumes of water. However, in the long term we will still need to consider the primary fuel source. While we currently strive to find an alternative to burning natural gas, there is much development taking place to seek a fuel that can flow through our national infrastructure. We may find that a breakthrough is achieved in the use of bio gas or maybe hydrogen – we read and watch with anticipation as these developments progress.
One answer for the CSSD may be through increasing electrification, by using electric clean steam generators. The electrical power can be obtained from a renewable ‘low/zero carbon’ source and this may solve some of the issues; many hospitals are indeed investing in their own solar capabilities. However, the thermal requirement of a number of sterilisers would place a significant instantaneous demand on the electrical infrastructure of a CSSD or Hospital. We touched earlier on the peaky profile of the steam load.
There is a solution to help overcome the need for large electrical loads. High instantaneous steam demand can be met using a steam accumulator, which can store the thermal energy that is produced by the generator, only to release it in the form of steam, as and when the process demands it. This can indeed reduce the peaks on electrical load, whilst satisfying the peaks of the steam demand.
A step on from this is to store energy, particularly from renewables like solar and wind, when they are available, or from the grid when tariffs are preferential. This harnessed energy can then be released to deliver steam to the sterilisers when needed. The challenge is to do this efficiently and with a technology that will minimise the environmental impact, both in its initial production and its end of life disposal. Spirax Sarco is using its expertise to produce innovative solutions relating to storing and delivering thermal energy which is harnessed from low carbon sources. This may well pave the way not only for sterile services, but for many aspects of heating and process in the future.
Who would have thought, the creation of low carbon steam? A real modern day, 21st century industrial revolution.
[1] Patient Safety NHS Improvement Hub https://improvement.nhs.uk/improvement-hub/patient-safety/
[2] British Medical Journal 2020 Public Health Original Research Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. Available at https://bmjopen.bmj.com/content/10/1/e033367[3] NICE Healthcare-associated infections Quality Standard 2016. Available at www.nice.org.uk/guidance/qs113
[4] HSE Methods of Contamination Guidance. Available at https://www.hse.gov.uk/biosafety/blood-borne-viruses/methods-of-decontamination.htm
When trying to trace the cause of wet packs on existing plant, this is something that is particularly difficult to assess. The common dryness testing regime is based on a point in time and is not continuous, so identifying wet steam can be somewhat ‘hit and miss’ and dependant on operating conditions prevailing at the time of the test. This latest generator can therefore safeguard against issues resulting from poor steam quality at all points in the operating cycle.Angelo Giambrone, Business Development Manager Best Practice Guide to achieving reliable and consistent high quality steam
Sterilisation in Healthcare
Could your hospital's sterilisation be more reliable and consistent every time? Find out how your sterilisation department could benefit
The evolution of steam and what does 'good' steam look like
Steam is an incredible heat transfer medium and, it’s come a long way from its traditional associations with locomotives and the Industrial Revolution. Today it’s an integral, clean and essential part of modern technology. Without it, our food, textile, chemical, medical, power, heating and transport industries could not exist or perform as they do.
Steam Quality in Food and Beverage
Are you using the correct grade of steam in your process? Using an inappropriate grade of steam for your process(es) can be a source of contamination.
5 ways to optimise your healthcare steam system
We list the 5 ways you can optimise your healthcare steam system
Efficient steam trap management: How to maintain a safe, lower carbon and energy-efficient steam system
Implementing an effective steam trap management plan, doesn’t need to be complicated. Lowering Carbon output, increased production and energy savings are all benefits you could achieve from regular management.
Case Study: Plate heat exchanger packages deliver energy savings
Plate heat exchangers from Spirax Sarco are saving energy, reducing maintenance costs and improving the comfort of patients at Leighton Hospital.